

Permanganate ion in alkaline solution oxidizes Na 2SnO 2 to Na 2SnO 3. The balancing partial equations of the dichromate side and iodine side areįor balancing overall equations iodine side multiplies by 3 and adding with dichromate side.Ĭr 2O 7 -2 + 14H + + 6I – → 2Cr +3 + 3I 2 + 7H 2O Balancing Permanganate ion in alkaline solution MnO 4 – + 8H ++ 5Fe +2 → Mn +2 + 5Fe +3 + 4H 2O Balancing Potassium iodide in acid solution of dichromateįor balancing oxidation of potassium iodide in an acid solution of dichromate, chromium reduces from +6 state to +3 state, and iodine ion oxidized to form the elementary iodine molecule.

Therefore, to balancing charges of partial equations, this equation multiplies by 5 and then adding with the first equation. This equation balancing with respect to charges and reacting elements. The partial equation representing the oxidation of the reductant, Fe +2 ⇆ Fe +3 + e. The above partial equation is still unbalanced from the viewpoint of charges and the equation balancing by bringing in five electrons. This reaction occurs in an acid solution, thus we use hydrogen ion to balance the four oxygen atoms in permanganate ion. The partial equation representing the reduction of the oxidant, MnO 4 – → Mn +2. The left-hand side of the ultimate equation will carry, MnO 4 –, H + ion, and Fe +2 ion, and the right-hand side will have Mn +2, water, and Fe +3. Permanganate ion is the oxidant and the ferrous ion is the reductant in balancing chemical equations.
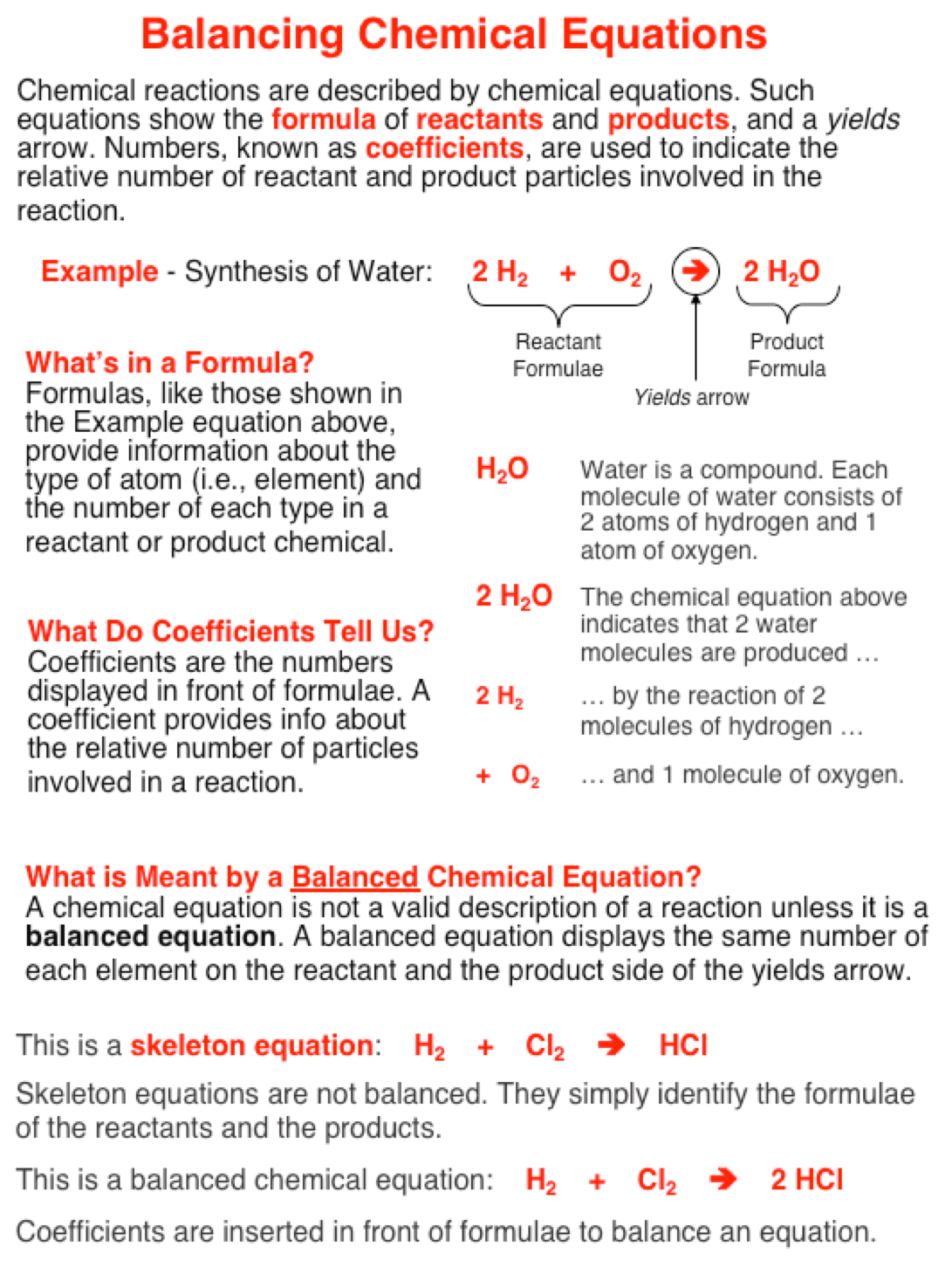
Potassium permanganate in dilute sulfuric acid oxidizes iron from the ferrous state to the ferric state by changing the electronic configuration of the iron atom.
Balancing the changes of partial chemical equations by adding a suitable number of electrons.If the reaction occurs in acid solution or low pH solution use a requisite number of hydrogen ions for balancing the number of the atom involved in the partial equations and for the alkaline solution or high pH solution we use hydroxyl ions.Set up the partial balancing equations representing the reduction of the oxidant, and the oxidation of the reductant.Certain the oxidizing agent and reducing agent, and their chemical formula for balancing chemical equations.We use rules for balancing equations by the ion-electron method are Balancing redox reactions by ion electron methodīalancing chemical equations by the ion-electron method has certain general rules or formulas which use for balance oxidation-reduction reactions or redox reactions.


 0 kommentar(er)
0 kommentar(er)
